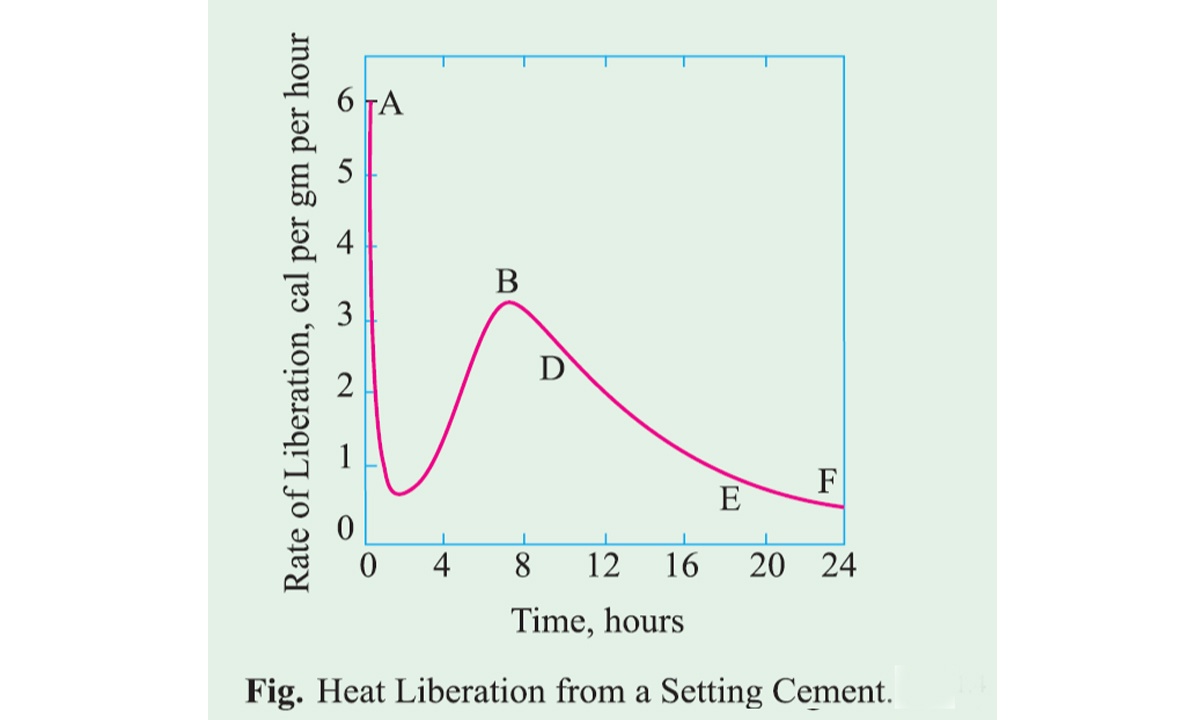
Hydration of Cement
Dry cement does not bind with fine and coarse aggregate. It acquires binder properties only when mixed with water. After mixing water in the dry cement a chemical reaction takes place between cement and water which is referred to as hydration of cement.
The chemistry of the concrete is essentially the chemistry of the reaction between cement and water. As a result of hydration, some products are formed which are very important because they have cementing and adhesive value. The quality, quantity, continuity, stability, and the rate of hydration products are important factors.
Anhydrous cement when mixed with water, reacts with each other to form hydrated compounds of very low solubility.
The hydration of cement can be visualized in two ways:
The first is through the solution mechanism. In this mechanism, the cement compounds dissolve to produce a supersaturated solution from which different hydrated products get precipitated.
The second possibility is that the water attacks cement compounds in the solid-state converting the compounds into hydrated products starting from the surface and proceeding to the interior of the compounds with time.
It is possible that both through the solution and solid-state types of the mechanism may occur during the course of reactions between cement compound and water.
The former mechanism may predominate in the early stages of hydration in view of large quantities of water being available, and the latter mechanism may operate during the later stages of hydration.